I am creating artificial life, declares US gene pioneer
Craig Venter, the controversial DNA researcher involved in the race to decipher the human genetic code, has built a synthetic chromosome out of laboratory chemicals and is poised to announce the creation of the first new artificial life form on Earth. ( The Guardian)
Monday, October 8, 2007
Nobel Season and a note on RNA
Interesting to note:
The Nobel in Medicine has gone this year to the inventors of gene-knockout techniques for mice, which seems well-deserved, considering how much has been learned through such experiments. This is, in fact, one of those discoveries that you'd think was already recognized by a Nobel if you hadn't been keeping count, which is as good a criterion as any. (It's rather odd, for example, that gene knockouts were recognized after RNA interference, don't you think, since a good ten or fifteen years separate the two in real life?)
My previous post noted that over 46,000 hits came up for "knock-out mice" search on PubMed. Interesting, RNAi gets only 9868, Certainly not a perfect measures but informative on some level.
Nobel Season. In the Pipeline:
"... Wednesday morning is the announcement of the Chemistry award, so I'm throwing open the gates of speculation, as I do every year around here. Our track record (mine and the predictions in the comments) has not been very good, but nobody has a good batting average when trying to read the minds of the Nobel committees. I feel pretty safe in saying that this year will be a "real" chemistry prize - we're one out of the last four, compared to overflow from the nonexistent Nobel in molecular and cell biology.
So, who's it going to be? Last year's uninformed gossip is here, and there's plenty more over at Chembark. Put your bets down, but only with money you can afford to lose. . .
Update: Still more speculation."
Three Americans Win Noble Prize for Medicine
work in gene targeting, a process using homologous recombination to
turn off a single gene. When used in mice it is known as using
"knock-out mice", as that gene has been knocked out. Having a single
gene, and only that gene, turned off helps to understand how that gene
functions.
The two Americans are Mario R. Capecchi and Oliver Smithies. Sir
Martin J. Evans is British.
To give an idea of how important this technique is today, a search on
pub med, the medical research literature database, for "knock-out
mice" gives you over 46,820 research papers.
Monday, September 10, 2007
New Cure for Depression: Fear (DrugWonks)
Public Perception Of Biotechnology
Find these answers in the latest issue of Biotechnology Journal, devoted to 'Talking Biotech with the Public' which will be entirely FREE for download during the month of September 2007, at http://www.biotechnology-journal.com/. "
SignOnSanDiego.com > News > Business -- Technology giants, banking firms win major victory with House approval of patent reform bill
Opponents of the bill included pharmaceutical and biotechnology companies, such as Johnson & Johnson and Amgen Inc., who say it weakens patent protection by reducing infringement penalties and permitting post-approval challenges to patents. ...
The Senate Judiciary Committee approved a similar version of the bill in July and it awaits a vote in the full Senate.
That bill includes a provision that would protect banks from damage awards in patent infringement cases involving electronic check-clearing technologies, such as check-imaging systems.
Banks have paid millions of dollars to settle allegations of infringement of such technologies, the American Bankers Association said in July.
"Patent Docs: Patent "Reform" Bill Passes House of Representatives
Off Label Juice
I've also noticed a lot of college kids buying it to cure hangovers. Who knew.
"As beverage giants add vitamins, electrolytes, bright colors and flavors to their bottles in search of the next best-seller, Abbott Laboratories has won some converts — without even trying. Scores of amateur and professional athletes are hooked on Pedialyte, a liquid meant to quickly rehydrate toddlers experiencing diarrhea, the New York Times reports . The fluid, which comes in bubble gum and other kid-friendly flavors, contains the basic ingredients in most sports drinks. Abbott doesn’t market its product as a sports drink, nor does it track its sales to athletes, but company doctors say they’re aware of off-label locker-room use and that Pedialyte does work on the field. “It’d be different if they were drinking formula,” the Minnesota Vikings’ coach told The St. Paul Pioneer Press last year. “But Pedialyte is used in hospitals throughout the United States for hydration. It’s different than just your regular sports drink.”"
Friday, September 7, 2007
Health Business Blog � Blog Archive � No biogeneric legislation this year
Biogeneric legislation is not happening just yet. And Health Business Blog adds:
"I’m hopeful that the biogeneric push –which apes the path taken for generic versions of traditional drugs– will be rerouted. The newer proposals are not terrible, but my preference is for price regulation of biotech drugs post-patent expiry. No biogenerics or “biosimilars,” no new clinical trials, FDA inspections and reviews. Rather, same drugs, same factories, same processes, lower prices."
Wednesday, September 5, 2007
RGJ.com: Popcorn maker changes butter chemical
The smell of movie popcorn. Seems so unfair.
Tuesday, September 4, 2007
All Mapped Out - Forbes.com
So where does this leave Ventor's patent on a gene chassis?
Health Business Blog � Blog Archive � Are we really 99.9 percent identical? No.
Putting some perspective on recent genetics news.
Sector Glance: Biotech Stocks Rise - Forbes.com
"Porges also said the regulatory environment, despite some high profile safety driven labeling changes, is relatively benign for biotech companies.I'm not sure I agree with that, but we'll see.
'Biotech companies tend to be developing and marketing treatments for serious, often life-threatening illness. Such treatments are not subject to the same risks of rare serious side effects in broad usage,' he wrote."
Tuesday, July 24, 2007
biosink: www.kansascity.com | 07/23/2007 | Kansas City lags in biotechnology investment
Kansas City-area leaders recognize the challenge that companies in this region face when trying to obtain money for refining their technology, hiring executives or making other moves to advance their business.'"
One wonders what the country will look like when all of these local biotech encouragement efforts kick in.
FuturePundit: Low Cholesterol From Statins Slightly Boosts Cancer Risks?
"I hear Joe Jackson singing:
Everything
Everything gives you cancer
Everything
Everything gives you cancer
Theres no cure, theres no answer
Everything gives you cancer"
I haven't read this study, but it seems to me that ALL THE TIME studies showing correlative data are reported with a "serious chance of causality" sort of tone. That plus the frequency of such stories are undermining people's interest in paying attention to cancer health.
Thursday, April 26, 2007
The Volokh Conspiracy - Regulatory Reform:
"Economists have long observed that regulators at, say, the Food and Drug Adminstration face unbalanced incentives. When people die because an unsafe drug got FDA approval, everybody blames the commissioners. When people die because a potentially lifesaving drug never made it to the marketplace (or was never developed in the first place because of costly regulations), the FDA's role is largely invisible. Therefore the commissioners are biased toward excessive caution."A problem to be worsened by what will surely be riskier follow on biologics approval?
Wednesday, April 25, 2007
Pharmalot: Kennedy Circulates Biogenerics Bill
"Ted Kennedy is reported to be circulating draft legislation which would provide an approval pathway at the FDA for generic versions of biotech drugs. His proposed bill would also provide a period of market exclusivity for brand-name biotechs before generic versions of their products could come to market.Still curious to see who takes the fall when we have the biotech generic equivalent of a Vioxx scandal. I don't think the supporting legislators who create the pathway should be off the hook.
The Democrat, who chairs the Senate Help, Education, Labor and Pensions committee, began circulating his proposals this month after the committee voted 15-5 in favor of the Food and Drug Administration Revitalization Act (S 1082), which he co-sponsored with the panel's ranking member, Republican Senator Mike Enzi.
That bill would reauthorise the Prescription Drug User Fee Act, which expires on September 30, for another five years, and increase by $50 million the amount which has been agreed between the FDA and industry to fund drug safety activities. It would not, however, create a biogenerics approval pathway at the FDA, although Kennedy forecast after the vote that this would be added before the bill goes to the Senate floor for a full vote, which he expected would be some time in June."
Tuesday, April 24, 2007
More Local Biotech
Nevada Development Authority (NDA) has selected Boston for its next targeted campaign in its ongoing effort to recruit life science and bio tech companies in the region. NDA will be exhibiting at Boston's BIO 2007 International Convention from May 6-9 at the Boston Convention & Exhibition Center. It is NDA's goal to educate the approximate 20,000 convention attendees on the growing life science and bio tech infrastructure in Las Vegas while promoting the substantial benefits of a favorable tax climate, overall low costs of doing business and less bureaucratic, business-friendly state and local governments.
Ironically Nevada was even more business friendly when Vegas politics dominated the state less than they do now. But it's all relative.
Biogenerics, Now In The House
Reps. Jay Inslee (D-Wash.), Gene Green (D-Texas) and Tammy Baldwin (D-Wis.) on Thursday introduced legislation ( HR 1956) that would allow FDA to approve follow-on biologics, CQ HealthBeat reports. Follow-on biologics are lower-cost versions of biotechnology drugs. FDA has no approval process for follow-on biologics. Under the measure introduced Thursday, drug makers would receive 14 years of data exclusivity for new follow-on biologics, with the possibility of an additional year if the HHS secretary approved the drug for a new indication that offered a "significant clinical benefit" during the 12 years following its authorization. The measure would allow the HHS secretary to issue guidance describing the data that would be required for approval of follow-on biologics in a particular class. The measure also would allow FDA to make a science-based determination about the data and information needed for approval of such drugs, including factors such as complexity and immunogenic reactions of the original product. In addition, FDA would be permitted to request comment from patient groups, brand-name drug manufacturers and generic manufacturers throughout the approval process. To ensure the safety of such drugs, FDA would be allowed to request nonclinical studies and clinical trials "at appropriate levels," according to the legislation.
The Patent System
Vonage, a leading Internet telephony company, appears before a federal appeals court today to argue that it should be allowed to continue signing up new customers while it appeals the recent verdict that its products infringe three of Verizon's patents. A Virginia jury last month ordered Vonage to pay Verizon $58 million for infringing the patents, and Vonage was barred from signing up new customers. Given Vonage's precarious financial position, a permanent ban on signing up new customers would effectively be a death sentence for the company.
The case raises some troubling questions about America's patent system, which seems to allow a deep-pocketed incumbent to drive an innovative competitor out of business. Vonage pioneered the Internet telephony market, and has enticed more than two million customers away from Verizon and other telephone industry incumbents. But while Verizon hasn't been able to stop Vonage's momentum in the marketplace, they've found more success in the courtroom.
Dendreon
Banc of America has some positive comments on Dendreon (NASDAQ:DNDN) saying they anticipate an approval decision on Provenge by May 15th, under the condition of completing the Ph III IMPACT trial, and expect potential stock price appreciation in the near term. This view is based on firm's recent interviews with an ex-FDA general counsel, a regulatory executive in a major pharmaceutical company and CBER panel members.
Pharmos: Mixed Trial Data
Drug developer Pharmos Corp. said Tuesday its postoperative pain treatment candidate showed mixed results in a midstage study.
The drug, an intravenous cannabinor, reduces pain versus placebo in a low-dose section of the Phase IIa clinical trial, at 12-milligrams, but failed to do so in higher doses of 24-milligrams and 48-milligrams.
"This is an unexpected pattern of results and the company continues to explore possible explanations," Pharmos (nasdaq: PARS - news - people ) said in a statement.
Shares of Pharmos rose 52 cents, or 35 percent, to $2.02 on the Nasdaq Stock Market (nasdaq: NDAQ - news - people ) in morning trading. The stock has traded between $1.36 and $2.58 over the last 52 weeks.
China Biotech
Amarin Shares Down
Amarin Corporation Says Huntington's Disease Drug Failed in Trials
LONDON, April 24 -- Amarin Corporation plc ("Amarin" or the "Company") today announces top-line results from its two Phase III clinical trials of Miraxion to treat Huntington's disease (HD). The Company conducted two Phase III double-blind, placebo-controlled studies in which HD patients were randomized to receive either placebo or 2 grams (1 gram twice daily) of Miraxion daily for six months. Study data showed no statistically significant difference in either study between Miraxion and placebo with regard to the primary and secondary endpoints.
These top-line findings are inconsistent with earlier clinical trial data that showed statistical significance in a subset of HD patients with a CAG repeat length of less than or equal to 44.
The primary endpoint of the trials was a change in the Total Motor Score 4 (TMS-4) component of the Unified Huntington's Disease Rating Scale (UHDRS). TMS-4 has been shown to be a sensitive measure of movement disorder in patients with HD. In addition, secondary endpoints included cognition and Total Functional Capacity outcomes. Miraxion was found to be safe and well-tolerated by patients.
Commenting on today's announcement, Rick Stewart, Chief Executive Officer of Amarin, said, "We are extremely surprised and disappointed by these top-line results, and we are analyzing the data in order to better understand the full and complete data set and outcomes. We are particularly disappointed that, at this time, we are not in a position to bring any positive news to those patients who are suffering from this devastating disease and to the broader HD community."
Celeb's at BIO
"Biotechnology industry leaders from around the world will hear from an exciting selection of world renowned keynote speakers during the 2007 BIO International Convention being held at the Boston Convention & Exhibition Center from May 6-9.Keynote speakers include founder of the Michael J. Fox Foundation for Parkinson's Research, Michael J. Fox, Her Majesty Queen Noor of Jordan, and U.S. Senator Edward Kennedy. Each speaker will provide insights on key biotechnology-related topics with respective addresses focusing on translating research into therapies, global health, and pressing public policy issues."
more...
Ausy Biotech
AstraZeneca to buy MedImmune
London-based pharmaceutical giant AstraZeneca plans to acquire MedImmune, the region's hallmark biotech company, in a $15.6 billion cash deal expected to close in the next two months.
Neurochem Playing Defense?
Couched in Thurday's press release regarding the study was the announcement that Neurochem's statisticians were making an "adjustment to the initial statistical model, as set out in the statistical plan, (that) would be necessary to provide accurate results."
Yikes! Nothing about that statement appears positive in any way.
U.S. Venture Cap up 8 Percent to $6.96 Billion
California dominated the venture capital activity in the first quarter, representing 44% of the nation's deal flow and 48% of the capital invested. The report showed that by major regions:
* The San Francisco Bay Area remained a stronghold for venture capital with 189 deals and $2.20 million invested. Although this is seven fewer deals than completed in the first quarter of 2006, the capital investment increased 10%.
* For the third quarter in a row, Southern California saw more capital investment than New England. In total, $1.10 billion was invested in 66 deals. This is the first time Southern California investment has topped $1 billion in a single quarter since 2000.
* New England remains the second most active region of the country in terms of deal flow, with 73 completed financings, which raised $927.9 million.
* The New York metropolitan area declined slightly, with $320.1 million invested here in the first quarter, down 38% from the same quarter of 2006. Deal flow, at 39, was basically flat with the same period.
* Investment in Washington state increased 21% over the same quarter a year ago to total $397.1 million in 27 deals.
* Capital investment more than doubled in the Research Triangle region although deal flow was flat, with a total of 11 deals and $202.8 million invested in the first quarter.
* Texas also posted an increased in capital investment, by 17% to $280.8 million, although there were seven fewer deals than in the first quarter of 2006.
Thursday, March 29, 2007
Merck Wins Patent Extension Case
Aaron F. Barkoff submits: The U.S. Court of Appeals for the Federal Circuit held today (pdf file) that a patent term extension under 35 USC 156 may be applied to a patent subject to a terminal disclaimer under 35 USC 253, handing a victory to Merck (MRK) in its battle with Hi-Tech Pharmacal (HITK) over generic Trusopt (dorzolamide HCl opthalmic solution).
R&D Crisis
In the past decade the pharma industry has experienced an innovation gap crisis characterized by relatively flat growth rate in new drug approval rates and a steady growth of over 2.5X in cost. Big pharma has tried to tackle the innovation gap with a series of strategic solutions: throwing money at internal R&D, horizontal consolidation, and increased in-licensing from the biotech sector, all with limited success. In our whitepaper, we survey the literature to pinpoint the fundamental root causes of the innovation gap and explore some of the emerging business models and solutions on the horizon and assess their opportunities and challenges in addressing the R&D crisis.
Thursday, February 22, 2007
Monday, February 12, 2007
Revenue a laughing matter -- for now / Err
Maybe by the end of the second day of the scientists and business people were just punchy, but every speaker seemed to have a joke to tell last week at the Florida Venture Capital Conference in Boca Raton.
Dam well better! Lol @ punchy.
.. Ernst & Young's Rich Ramko kicked off his talk with a definition tailored for the audience.
Q: What's a biotech company?
A: A pharmaceutical company without revenues.
Hey Ooooooo!
Actually, the biotech industry is becoming increasingly more stable, Ramko said. "We see an explosion in biotech in the next five years or so."
Love it or leave it. Here's my favorite part:
"It's all happening and it's happening in an incredibly short time."
Hold your breath!
The End is Nye!
U.S. scientists are using statistics to identify genetic social interaction traits among animals, thereby finding more productive livestock.
Woops. Sure it starts with cows, but homo Sapiens can't be far behind. More:
Researchers from Purdue University, the Netherlands and England designed mathematical equations based on traits to choose animals that are more congenial in groups, said William Muir, a Purdue geneticist. The new method is a tool that might contribute both to animal well-being and to securing the world's future food supply, including possibly permitting more animals to be domesticated, Muir said.
He said the tool makes it possible to design selective breeding programs to effectively reduce competitive interactions in livestock. It also aids in predicting how social interactions impact the natural evolution of species.
I never did like that bar on east 45th street...
This Just In!
Plants are being modified to deliver anti-oxidants, which protect against cancer; lipids, which contain essential fatty acids that serve as energy sources; vitamins, such as beta-carotene or vitamin A, which protect against premature blindness and susceptibility to other illnesses; and iron, whose deficiency results in fatigue and decreased immunity, she said.
Bananas and tomatoes are being engineered to deliver, among other things, antibodies for E. coli bacteria-induced diarrhea, a major killer of children around the world. Other plants are being engineered to counteract allergies, Newell-McGloughlin said.
So far, the United States has approved more than 70 genetically modified crops. These crops, which can be grown commercially, include canola, papaya, potato, rice, squash, sugar beets, tomato and tobacco, which is used to help produce a vaccine that fights against a type of lymphoma, said Newell-McGloughlin.
Research is being directed to making already healthy foods, such as protein-rich soy and soy oil with low or no saturated fats, taste better to consumers, Chassy said.
Also being developed are bioengineered trees capable of absorbing harmful chemicals from the soil and plants that can be converted into plastics and industrial products, he said.
At the International Rice Research Institute (IRRI) in the Philippines, researchers are developing plants that are "phytosynthetically more efficient." These have more leaf surface exposed to the sun, making the leaves more efficient in converting carbon to energy for higher yields, according to Carlos Quiros, a professor and geneticist at the University of California-Davis.
It's the first of a two part article. Also:
Although considerable research is being conducted by governments, international organizations, foundations, companies and academic institutions, few new products are being commercialized, the scientists said.
They explained that the many, separate country regulatory and patent dispute processes that often are lengthy and costly discourage commercial production. Several of the researchers called for a worldwide regulatory regime.
Also affecting the pace of commercialization is resistance from some consumers to accept that bioengineered foods have been proven to be safe, the scientists said.
Incentives and attitudes seem to rule the land.
Back-room Dissections
As the scandal unraveled in 2005, prosecutors revealed that Mastromarino had netted $4.6 million in three years of back-room dissections. He paid undertakers $1,000 a pop for providing access to the dead, paid cutters $300 to $500 for extracting the most marketable parts, and, according to his lawyer, managed to take home up to $7,000 per body. (One of Mastromarino's former employees contends the boss was pulling in double that.) The New York Police Department later interviewed the families of 1,077 people whose bodies were raided for spines, bones, tendons, and other tissues. BTS had cut deals with funeral homes in New York City, Rochester, Philadelphia, and New Jersey.
Cooke's bones were sold to Regeneration Technologies, one of the country's largest tissue banks. The company says Cooke's bones were deemed unsuitable for implantation, but it can't say the same for other pieces of tissue it bought from Mastromarino. The tissues BTS distributed ended up everywhere from a woman's neck in Kentucky to a man's jaw in Tampa Bay. Hundreds of people wake up every morning knowing that they are partly composed of stolen body parts.
In February 2005, Mastromarino and three others were indicted on 122 charges, including body stealing and opening graves. The grisly story received perhaps more media attention than any such scandal since a wave of body snatching in the 18th century. A February 2006 Paula Zahn Now segment spun the story into the perfect media narrative, complete with a villain, a celebrity, and a whistleblower. But that telling, and many others, failed to point out that much of Mastromarino's basic business model was perfectly legal, common, and necessary to the biotech industry. If Mastromarino had been smarter, he could have made a fortune off body parts while staying well within the limits of the law.
Law and custom both prohibit the sale of cadaveric tissue, a ban heartily supported by bioethicists like Arthur Caplan, the influential director of the University of Pennsylvania's Center for Bioethics. The prohibitionists warn of the degradation and commodification of human beings, but scientific progress has blurred the line between tissue and commodity. Doctors need a constant stream of remains to perform-and profit from-their work. The current compromise treats the body as property once it's in the hands of a corporation but as a "priceless" gift as it passes from a donor's family into the marketplace.
Where does the recovered tissue end up? According to Cindy Speas, WRTC's director of community affairs, the organization is "not involved in any way with anything that is not a not-for-profit." And it's true that the consortium doesn't send tissue directly to corporations. Instead, WRTC provides tissue to LifeNet, another nonprofit, whose mission is to "improve the quality of human life" and "serve the community." LifeNet posted $107 million in revenues in 2004 for "tissue/organ procurement, processing fees, and reimbursements."
From LifeNet, the tissue enters the for-profit system. LifeNet has contracted with LifeCell, the company that makes AlloDerm, along with other "alliance partners" such as Osteotech, the firm that makes bone putty. Standard and Poor's lists LifeCell's value at $888 million. >From there, tissue can end up as replacement skin for a young burn victim or cosmetic filler for a thin-lipped socialite.
Here's the crux of it:
Six years ago, two journalists at The Orange County Register undertook the most extensive investigation to date of the legal tissue trade. They linked 59 nonprofit tissue procurement agencies with publicly traded, for-profit firms. They also called each agency for comment, and the recorded answers are a jaw-dropping chronicle of deception and arrogance. The director of the nonprofit California Transplant Donor Network, which at the time was selling bone to Osteotech, admonished the Register, "It is not legal to sell organs and tissue." Others explained that families could not comprehend the distinction between nonprofit and for-profit. A spokesperson for the University of Miami Organ Procurement Agency, which sells skin and bone to the biotech company CryoLife, explained, "We can't be educating donors at the bedside."
It's a tough trade off between creating a demand for human tissue that is directly felt by people or pretending that demand doesn't exist and cutting them out of the profit loop.
More on Local Biotech: Arizona
They spent months laying the groundwork for a Scottsdale Airpark-based investment bank and advisory firm that they hope will grow with the region's budding biotech industry.
Alare Capital Partners LLC brings together 12 founding partners with backgrounds from pharmaceuticals to mergers and acquisitions to technology licensing. Scientific and business advisory boards add 11 more experts to help generate business leads and provide expertise in technical areas.
"I think it would have been difficult to do two to three to five years ago," Rodgers said of founding the specialty firm in a Valley where other banks didn't last. "There was a sense that there wasn't enough critical mass here, that it was a golf-and-retirement kind of economy. But when TGen (the Translational Genomics Research Institute) and IGC (the International Genomics Consortium) came, that changed everything. It put Arizona on the map."
A lot of local governments like the externalities of biotech but don't always appreciate the role those apparent externalities played in growing biotech in the first place. For Arizona this is certainly a step in the right direction. See this older article:
Now California and the Boston metropolitan area are home to almost all of the few venture capital firms with expertise in funding biotechnology startups. And that, Royston said, poses a problem for the cities in between that are striving to develop their own biotech clusters.
"There's plenty of money out there, but not enough of the kind of venture capital that starts a company," Royston said.
About a year ago, a group from the University of Pittsburgh met with Royston to pitch its idea of spinning off a scientific discovery into a startup biotech company.
"I loved the technology," Royston said. "But in the end, I told them that it was going to require a lot of hands-on work by experienced investors to start the company. I told them to find someone in Pittsburgh to help. I told them that if they were in San Diego, it would be a different story.
The Biotech Blackhole
Xoma, which Dr. Scannon started in 1981, has never earned an operating profit or marketed a drug of its own. And in the quarter-century since its birth, Xoma has managed to burn through more than $700 million raised from investors and other pharmaceutical companies.
...
Biotechnology has been "one of the biggest money-losing industries in the history of mankind," Arthur D. Levinson, chief executive of Genentech, told analysts in New York last year. He estimated that the biotech industry as a whole has lost nearly $100 billion since Genentech, the industry pioneer and one of its most successful companies, opened its doors in 1976. Only 54 of 342 publicly traded American biotech companies were profitable in 2006, according to Ernst & Young.
I won't argue with these smart people haphazardly in this space, though I do think such statements have caveats. Perhaps I will discover them in the Pisano book.
Antibody Deal
AstraZeneca will use Regeneron's VelocImmune technology to discover human monoclonal antibodies. It will do the research work at Cambridge Antibody Technology, a British company AstraZeneca acquired last year. ...
Under the terms of the agreement, AstraZeneca will pay Regeneron $20 million upfront and make up to five annual payments of $20 million. It will also pay Regeneron a royalty in the mid-single digits on any products that come to market.
Saturday, February 10, 2007
Thailand Reconsiders Generics Move
Thailand backs off threat to break drug patents ( SciDev.net)
[BANGKOK] Thailand has delayed breaking the patent of an AIDS drug and a heart medicine, and entered into negotiations with drug firms to lower the price so that more people can be treated.
Friday, February 9, 2007
Biotech Cost Ceiling II
The new Pharmacor report Crohn's Disease finds that third-party payers
in both the United States and Europe impose significant limitations on the
use of biologics to treat the disease. One of the limitations in most of
the major pharmaceutical markets is that patients must fail to respond to
at least two conventional therapies, such as corticosteroids and
immunosuppressants, before receiving treatment with a biological agent. As
a result, only a small percentage of Crohn's disease patients will be
treated with biologics during the 2005-2015 forecast period.
More on Adult Stem Cells
Johns Hopkins Kimmel Cancer Center scientists have found a set of "master switches" that keep adult blood-forming stem cells in their primitive state. ...
The scientists located the control switches not at the gene level, but farther down the protein production line in more recently discovered forms of ribonucleic acid, or RNA. MicroRNA molecules, once thought to be cellular junk, are now known to switch off activity of the larger RNA strands which allow assembly of the proteins that let cells grow and function."Stem cells are poised to make proteins essential for maturing into blood cells, but microRNAs keep them locked in their place," says cancer researcher Curt Civin, M.D., Ph.D., who led the study. The journal account will appear online the week of February 5 in the early edition of the Proceedings of the National Academy of Sciences.
Cancer Gene News
US scientists have cracked the entire genetic code of breast and colon cancers, offering new treatment hopes.
The genetic map shows that nearly 200 mutated genes, most previously unknown, help tumours emerge, grow and spread.
The discovery could also lead to better ways to diagnose cancer in its early, most treatable stages, and personalised treatments, Science magazine reports.
The Johns Hopkins Kimmel Cancer Center says the findings suggest cancer is more complex than experts had believed.
Stem Cells: Progress Marches On Either Way
Regarding stem cells I recently highlighted an angry letter from a reader of a local news source in Louisiana that made some good and often overlooked points:
The Bush administration was the first to fund embryonic stem-cell research and has devoted well more than $100 million to it since 2001, though only in ways that do not encourage the further destruction of embryos. Bush invokes his faith in explaining his position. ...
There is no ban in place preventing private companies from investing money to fund embryonic stem-cell research. If this research holds as much promise as Mr. Gautreaux feels it does, why aren't private companies lining up around the block to fund it?
To further the point, there's a site I previously linked to that continues it's coverage of private companies engaged in Adult Stem Cell research.
Fumento makes a good argument for adult stem cells and highlights the promise of the recent breakthrough amniotic stem cell harvesting.
There are over four million births each year in the United States, yet Atala calculates that merely 100,000 amniotic stem cell specimens could supply 99 percent of the U.S. population's needs for perfect matches for transplants.
We shall see if the impressive potential of amniotic stem cells comes through in the long run. Nonetheless it's safe to say that overall stem cell progress marches on:
Stem cellsI don't necessarily have an objection to embryonic stem cell research, or Bush's policy. But the issue certainly deserves accurate representation.In a grass roots effort to reject the Bush Administration's recalcitrant position on what promises to be the most exciting area of stopping and reversing - and possible curing - the progression of many major diseases, many states took legislative initiatives to accelerate the finding and development of new stem cell-based research and therapies.
Again, am I missing something in saying that the consequence (perhaps unintended) of Bush's embryonic stem cell funding ban seems to have been to simultaneously start a conversation about bioethics while not managing to stifle innovation?
more: A couple of afterthoughts. First, while I don't dispute the validity of adult stem cells, I'm not sure I dismiss the difference between pluripotent cells and multipotent cells as quickly.
And two, the debate around adult stem cells seems to be centered on declaring embryonic stem cells medically unnecessary as a way of settling or side stepping the ethical debate. I think the ethical debate of embryonic stem cells and the utility debate around adult stem cells are seperate issues that should be settled on their own merits.
Biogenerics and Antitrust Law?
"There's no way to 100 percent accurately reproduce the host that these compounds grow off of," said Casey Alexander, analyst for Gilford Securities. "Therefore it can never be functionally identical."
The only way for a biogeneric company to produce an exact copy of a brand-name biotech drugs, said Alexander, is to "sneak into the factory and to steal the host, which is illegal."
Is it possible that someday companies will be forced to license or turnover these hosts that produce off patent biotech drugs? I'm no expert in antitrust law but it seems to me there was a time when the Microsoft/Netscape case was unanticipated.
"Experts say the best opportunities for industry leaders Teva, and Barr are overseas and in biotechnology"
Barr also has an eye on the future in biogenerics, or generic versions of biotech drugs.
Biotechnology, a method of drug development that uses living organisms, was established in the 1970s with the debut of Genentech, the world's second-largest biotech in terms of sales behind Amgen. Biotech drugs have been in existence long enough for the patents to run out on some of the industry's older products.
But the FDA does not have the regulatory process necessary for the creation of biogenerics, meaning that biotechs in the U.S. do not have to compete against low-cost generics.
Missing the biogeneric boom
However, Rep. Henry Waxman, D-Calif., is trying to change that. Waxman became one of the most important figures in the drug industry in 1984, when he and Sen. Orrin Hatch, R-Utah, drafted the Hatch-Waxman bill that created the regulatory process for generic drugs in the U.S.
A staffer for Waxman said he plans to submit a bill in the next few weeks to lay the foundations for the biogenerics industry in the U.S. This is on top of another biogenerics regulatory bill that Waxman filed last year with Sens. Charles Schumer and Hillary Clinton, both Democrats from New York.
While biogenerics hasn't gotten off the ground in the U.S., it's up and running in China and Eastern Europe, where companies produce generic versions of biotech drugs. In 2006, Barr bought the Croatian company Pliva, the biggest drugmaker in Eastern Europe and a pioneer in the emerging but risky field.
Sawyer of Leerink Swann said in a published note that Teva and Barr are the "best positioned to benefit from the biogeneric opportunity" because of their involvement in the multinational markets, though the potential benefits from the U.S. market are four or five years away.
"My view is that by 2010 you'll see biogenerics in the U.S.," said Forman. "The U.S. will be the last country in the world to have biogenerics."
I'm in no way opposed to biogenerics. I am curious how the trade off between cost and safety will be resolved. I'm also curious why I never hear anyone pushing biogenerics legislation mention the potential of generic competition to significantly raise the price of biotech drugs before the expiration of the patent. In any case, protectionism never really works out very well.
There are other ideas out there, such as a granting a permanent monopoly to a drug inventor while controlling the price after a certain period of time:
This would avoid the costs and risks of biogeneric development and regulatory approval while delivering the benefits of lower costs to payers. The original maker of the product should be happy too. Although their price will be lower than it is today, they won't have to share the market with generic players or spend money blocking the entry of new players. They will still enjoy a substantial period of high margin sales as they do today. It just won't go on forever.
- Allow biotech drugs to be approved and marketed as they are now, without price regulation
- After patent expiration or after a certain number of years on the market, regulate price. The price could be based on cost of goods, a percent of the previous selling price, or some other mechanism
When, at some point in the future, science improves to the point where truly identical biogenerics can be developed, these rules could be revisited.
It probably would lower the overall price of the drug for consumers, however I'd be concerned that over time the permanent monopoly would lead to a substantial decrease in quality and perhaps innovation. However if the policy were only in place until technology advances to allow the FDA to truly determine sameness in biogenerics it's probably a decent idea, albeit DOA.
CSU's New Tech Transfer Approach
Colorado State University announced a new program, called MicroRx, which is intended to move infectious-disease research to market faster than existing technology-transfer models in the state.
CSU held a briefing about the concept Thursday at its office in downtown Denver.
MicroRx is the first of CSU's "superclusters" -- alliances of academic researchers, economists and business experts. The Fort Collins-based university began developing the superclusters concept in 2004.
As a nonprofit entity, MicroRx will focus on infectious disease, and biomedical research and development -- a niche that has gained CSU renown in recent years.
Thursday, February 8, 2007
The High Price of Drug Patents
In the meantime Congress has stepped in. On Jan. 17, Senator Herb Kohl (D-Wisconsin) proposed a bill that would make it "unlawful" for a settlement between a drug patent holder and a generic challenger to "include an exchange of anything of value." Expect major pushback from both generic companies and the notoriously powerful Big Pharma lobby.
Besides Congressmen, the FTC has another ally - Bernard Sherman, the Apotex CEO. Here's how he played out his "settlement" with Bristol and Sanofi: First, Sherman asked the patent holders for permission to launch generic Plavix right away should the FTC and state attorneys general turn the settlement down. (The regulators had that power because Bristol was operating under a consent decree stemming from past bad behavior over its patented drugs.) Bristol and Sanofi agreed and said generic Plavix could stay on the market for a window of five days in such an event.
Then Sherman's lawyers told the regulators that Bristol had offered Apotex unwritten side arrangements. The regulators rejected the settlement. They decline to say why, and Sherman says he thinks they would have rejected the deal regardless of the side arrangements.
Apotex immediately flooded the market with generic Plavix it had already made, selling a six-month supply before Bristol could get an injunction to stop it. Instead of the $40 million that it would have collected in the settlement, Sherman's company probably made multiples of that by actually selling the drug. In September, Dolan - long under fire - lost his job, at least partly as a result of this bungled deal.
The Justice Department has launched a criminal antitrust investigation into the settlement. Bristol denies that it ever made any unwritten side agreements. (It also says that the $40 million in the settlement would not have been a payment to keep generic Plavix off the market, but rather one to reimburse Apotex for what it had manufactured.)
Sherman won't actually come out and say that his settlement agreement was a ruse, but he has said that he extracted concessions from Bristol fully expecting that the regulators would scuttle it. He says Apotex has never settled a patent case: "Both generics and brands are trying to make more money at the public expense," he says.
This article sheds light on why generics companies are so eager to make deals for short term access to the market:
Generic drugmakers produce and sell drugs after patents expire. Generic drugs are most profitable in the six months following patent expiration, when the price only drops by about 40 percent because there is only one generic drugmaker producing it.
Generic companies fight over this six-month window of exclusivity, because the price drops another 40 percent when it runs out, cutting into profits.
Pharmaceutical Skirmishes
Here are some recent skirmishes that have seen developing countries protest against pharmaceutical companies' drug patents:
A decent roundup.
Wednesday, February 7, 2007
GM Crops
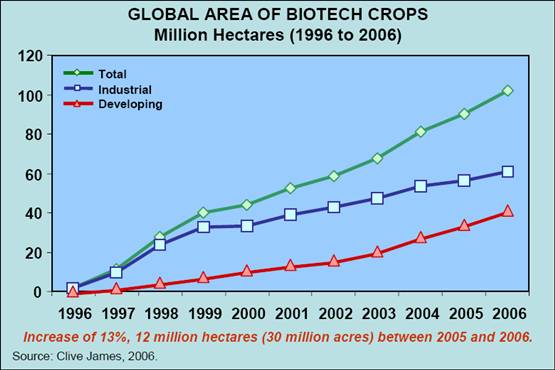
Salon.com:
In 2003, varieties of hybrid cotton seed known by the brand names Ganesh and Brahma were the popular choice among farmers in Gudepad, a village in the Warangal district of the state of Andhra Pradesh in India. By 2005, a majority of farmers throughout the entire district had switched to RCH-2 Bt, a genetically modified strain of cotton designed to produce its own insecticide.
The biotech industry would like us to believe that the migration to GM cotton was a result of individual farmers' testing out various seeds and coming to the sage conclusion that RCH-2 Bt performed the best. In contrast, anti-GM activists, even as they claim, despite the evidence in Warangal, that farmers are rejecting GM crops, also stress that whatever adoption does occur is likely the result of corporate propaganda and distribution muscle. Oh, and the new strains don't work as advertised, either.
But as is so often the case, neither side's explanation fully captures the situation on the ground.
Biotech, Pharma Outsourcing
There has been a lot of attention in recent years on the outsourcing of pharmaceutical research and manufacturing to India. While domestic pharma majors may be the beneficiaries of this trend, the original giants of outsourcing are not lagging far behind. IT behemoths like TCS and Wipro are grabbing a piece of the pharma outsourcing pie by getting involved in clinical data management. TCS recently secured a deal with Eli Lilly, which includes the establishment of a facility in Noida. Other pharma companies too have outsourced clinical data management operations to India including Pfizer, GlaxoSmithKline, Novo Nordisk and Wyeth.
And then this on biomedical opportunities in China:
There are over 2,000 Chinese-foreign joint ventures in the biomedical sector, including major players like Roche (RHHBY), Novartis (NVS), GSK (GSK), Pfizer (PFE), Medtronic (MDT), Becton Dickinson (BDX) and Inverness Medical (IMA), just to name a few. Most are significantly expanding their operations and are investing heavily in research and development to move beyond just manufacturing and distribution. China is encouraging and even subsidizing this migration, as it recognizes that to sustain its incredible historic growth rate of 9% per year, it must move its economy toward R&D.
BIO likes Bush
WASHINGTON--(BUSINESS WIRE)--The Biotechnology Industry Organization (BIO) today praised the far-reaching initiatives contained in the U.S. Department of Agriculture's 2007 Farm Bill Proposals to encourage the production of biofuels and biobased products from renewable agricultural resources, as well as ensure fairness of international trade for agriculture.
"The 2007 Farm Bill proposals show the Administration's support of biotechnology in both industrial and agricultural applications," said Jim Greenwood, president and CEO of BIO. "We greatly appreciate the Administration's demonstrated commitment to support companies researching and commercializing both ethanol from cellulose and biobased products from renewable agricultural resources. The proposals related to international trade illustrate the importance of internationally accepted regulatory standards, many of which affect biotech crops."
Bush & Stem Cells
The Bush administration was the first to fund embryonic stem-cell research and has devoted well more than $100 million to it since 2001, though only in ways that do not encourage the further destruction of embryos. Bush invokes his faith in explaining his position. ...
There is no ban in place preventing private companies from investing money to fund embryonic stem-cell research. If this research holds as much promise as Mr. Gautreaux feels it does, why aren't private companies lining up around the block to fund it?
There's certainly plenty of private research being done. Here's my previous post on some perhaps inadvertent benefits of the policy. I don't fully agree with the reader or the policy, but the discussion in the news is really annoying.
Inventive Step v. Obviousness
On February 5, 2006, the U.K. Patent Office published the final report on the its "Public consultation on level of the inventive step required for obtaining patents:
And something noteworthy from the comment section:
Anonymous said...
Tough Growing Drugs
Lincoln - The opportunity to grow medicine in cornfields may have slipped away from Nebraska and Iowa farmers for at least the time being.That'll do 'er!
The biopharming movement - the attempt to put genes into plants that will reproduce into therapeutic drugs - continues to advance, but companies are shying away from corn as a potential drug-making factory.
"I see it as a lost opportunity," said Aurora, Neb., farmer Richard Schaffert, who was among the Midlands farmers in 2002 who planted test plots of corn for a biopharming company called ProdiGene Inc. of College Station, Texas.
The crops-to-drugs industry suffered a major setback when gene-altered corn plants from the previous year's ProdiGene tests emerged among soybeans growing in the same fields.
High Feed Costs
In Latin American and the Caribbean (LAC) countries, in spite of the abundance of natural resources and continued investments in development, poverty and food insecurity affect more than 55 percent of the rural population. Fifteen years ago, plant biotechnology comprised only a few applications of tissue culture, recombinant DNA technology and monoclonal antibodies. Today, genetic transformation, and marker-aided selection and breeding are just a few of the examples of the applications in crop improvement with profound implications for the LAC Region.
Plant biotechnology applications must respond to increasing demands in terms of food security, socio-economic development and promote the conservation, diversification and sustainable use of plant genetic resources as basic inputs for the future agriculture of the Region.
Wyeth & Biotech Deals
The deal with Elbion focuses on discovering drugs to treat schizophrenia, while collaborations with Nautilus Biotech and MediVas are aimed at developing more advanced haemophilia treatments, either by developing new drugs or a better drug delivery system.
Saturday, February 3, 2007
Africa UN & Biotech
African Heads of State have endorsed a 20-year biotechnology action plan for the African Union, but held off committing to a science and innovation fund.
Agri Bio Report:
SAN FRANCISCO, FEB 2: A biotechnology advocacy group reported on Thursday that a record number of crops were planted worldwide last year, but critics complained the gains did not go beyond making corn, soy and cotton crops resistant to weed killers and bugs.
None of the genetically engineered crops for sale last year were nutritionally enhanced and much of the output feeds livestock, which critics said undercuts industry claims that biotechnology can help alleviate human hunger. Still, the report prepared by the industry-backed International Service for the Acquisition of Agri-Biotech Applications touted the record as evidence that crops engineered to cut pesticide use can ease poverty and financially benefit small farmers around the world.
A Peek at Ausy Biotech
According to investment bank EG Capital, the 121 biotech companies on the ASX have a total market capitalisation of $6.9 billion. That's equal to the market cap of just one US biotech company, Chiron (the fifth-biggest US biotech stock).
Curing pigs:
MAYBE we should have known that a company selling a cure-all for pigs and chickens might be risky. But I do feel sorry for anyone who bought the failed biotech wonder stock Chemeq in recent years — especially those who paid $8 a share back in 2004.
Because the way things are going, the last share trade at 21 cents is beginning to look a little on the high side.
The managers of Chemeq are holed up in Perth this weekend wondering how they can come up with $60 million they have been ordered to pay bond holders by the WA Supreme Court. It won't be easy — the total value of the company is $21 million.
Chemeq is not the first biotech company to lose heaps of money for shareholders and make a joke of the sector, and it won't be the last. But it's worth looking at why this company, which planned to develop and manufacture an alternative to antibiotics in farm animals, has bitten the dust. ...
Top 5 Reasons to Consider Biotech
For anyone interested in investing in, or thinking of pursuing a career in biotech, whether at a large company or a small startup, here are five positive indicators of how viable the industry is today. These signs speak volumes of the potential and popularity of biotech today among investors and politicians alike.
They expound on job satisfaction, fast growing job sector, available funding, foreign opportunities and growth potential.
Feuerstein's Biotech Mailbag
I mock Lexicon Genetics, er, Pharmaceuticals, but in all seriousness, why did this rejuvenation take so long? Human Genome Sciences (HGSI - Cramer's Take - Stockpickr - Rating) and Millennium Pharmaceuticals (MLNM - Cramer's Take - Stockpickr - Rating) learned the painful lesson years ago that spending your days poking around human DNA might be noble work, but it certainly wasn't a viable business model. The real money is in developing, and hopefully selling, drugs.
Biogenerics... in Iran.
02 Feb 2007 - Interferon-beta is used for multiple sclerosis therapy. An interferon-beta protein developed at the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB, Stuttgart, Germany, in collaboration with CinnaGen company, Tehran, Iran, is now the first therapeutic protein from a Fraunhofer laboratory to be approved as biogeneric / biosimilar medicine.
I'm not sure what the global biogenerics landscape looks like but perhaps we can learn from other countries experiences if they're trying it ahead of us.
Adult Stem Cells
Although human embryonic stem cells have greater potential than adult stem cells, embryonic stem cell research hasn't kept up pace with adult stem cell research. The biggest advantage of adult stem cell is it is free of ethnic controversy. The first adult stem cell based treatment will be on the market within next couple years, much sooner than embryonic stem cell. For the last few days, I tried to collect information for adult stem cell biotechnology and industry. I was very impressed by the progress they have made.
He starts with Osiris Therapeutics Inc.
Friday, February 2, 2007
Palo Alto biotech & Schering-Plough
In the latest of several recent lucrative deals between Bay Area life-science companies and major drug makers, Anacor Pharmaceuticals of Palo Alto said Friday that Schering-Plough has agreed to pay it up to $625 million for its toe-fungus treatment.
...
Last month, Amgen of Thousand Oaks in Southern California agreed to a deal worth up to $725 million to help develop heart-failure treatments with Cytokinetics of South San Francisco. Since 2004, Amgen also has purchased three Bay Area companies -- Avidia of Mountain View, Abgenix of Fremont and Tularik of South San Francisco -- for a total of $3.88 billion.
In October, Merck of Whitehouse Station, N.J., announced it was paying $1.1 billion in cash for Sirna Therapeutics of San Francisco, which had been developing drugs to combat cancer, the virus that causes AIDS and other diseases.
And in August, GlaxoSmithKline of London announced it would pay up to $1.5 billion for the rights to treatments being developed by Mountain-View based ChemoCentryx for bowel and inflammatory disorders.
LifeSpan BioSciences & Roche
Under the terms of the agreement, Roche Pharma will gain access to all the features of the CNS subset of the DrugTarget Database(TM). These include informatics on more than 3,400 genes, 886 comprehensive immunohistochemistry (IHC) reports with localization information on more than 450 potential drug targets selected by pharmaceutical company subscribers, and innovative search and analysis tools. LifeSpan continues to build the database by publishing localization data on an undisclosed number of additional protein targets each year.
FDA Reforms
The new Congress is coming out of the gate bristling with proposals for reforming the FDA and putting new reins on the biopharma industry. New measures would restrict drug advertising as well as create a new office of drug safety to oversee medications after they're approved. Similar measures have been floated before and never had a chance. But the new Democratic majority--allied with several senior Republicans angered by the agency's safety record--appear emboldened to act fast, which has industry officials somewhat alarmed.
The key concern among the trade groups is that the reformers are likely to piggy-back on the reauthorization of the user-fee bill, an essential piece of legislation for financing the FDA. Billy Tauzin (photo) at PhRMA says the bill could become a "Christmas tree" loaded with new measures. The drug industry is banking on a compromise pact with the FDA that increases user fees by about a third, up to $393 million next year. And they're hoping the extra money will help grease the tracks to get the bill through without reformers tacking on any new safety measures. Without a new user fee authorization the agency's drug regulation work will essentially be frozen by late summer, setting the stage for a major showdown in Washington.
More on biotech generics below.
Cytokine Drugs 20% of Market
CNBC Video on Dem's and Biogenerics
A look at how Congress may deal with the issue of biogenerics, with John Calfee, American Enterprise Institute Resident Scholar; Kathleen Jaeger, Generic Pharmaceutical Association CEO and CNBC's Sue Herera
I had questions about who's responsible for safety issues around biogeneric variability. So did John Calfee:
"I think that given the pressure the FDA is under for drug safety, the FDA is going to move very slowly to allow anything purporting to be a generic version of those drugs even if the law is changed opening the way for that kind of thing. And I think that doctors are going to be very slow to switch to those drugs..."
Kathleen Jaeger side steps the issue by saying the process should be ruled by "science." I think science is the hurdle for biogenerics, at least until technology advances.
Thursday, February 1, 2007
Biotech or Pharma?
In an earlier article, I reviewed Pisano's book Science Business: the Promise, the Reality, and the Future of Biotech, in which the Harvard business professor provides compelling evidence showcasing the ineptitude of the biotech industry over the last three decades.
But a recent report by the Government Accountability Office (GAO) paints a different picture all together. In the report, for which data was collected from the Pharmaceutical Research and Manufacturers of America, the GAO found that the pharmaceutical industry was in fact losing to the biotech industry in the number of new molecular entities (NMEs) approved by the FDA over the last few years.
2003 was the first time that biotechs had a higher number of NMEs approved, and the gap has widened since then. Pharma companies had 16 approvals that year, compared to biotechs' 18.
...
One major finding of the GAO report however had nothing to do with competition from the biotech industry. From 1993 to 2004, the report found that while R&D spending by the pharmaceutical industry increased by 147%, drug approvals only increased by 38%.
Thailand's Generics Move
While producing generic equivalents of these drugs might (it's still somewhat of a gray area) technically not be illegal -- Thailand is a member of the World Trade Organization, and developing countries have until 2016 to implement protections for pharmaceutical patents -- the country is still obligated to pay and negotiate licensing fees for the drugs, or else risk having a trade complaint filed against it.
Interestingly enough, discouraging pharmaceutical innovation with compulsory rules forcing the price of drugs down doesn't really help countries save on drug costs. The FDA has a white paper that shows that prices of generic drugs are cheaper in the U.S. than their Canadian-branded and generic counterparts, even though the U.S. gives stronger "incentives for R&D" spending.
Option and Licensing Decisions Hinge on Firms' Capabilities and Motivations
Tailoring options to account for firm differences, says Arvids Ziedonis, assistant professor of strategy at the Ross School, can facilitate the licensing of university inventions to industry yet protect against suitors who seek to absorb knowledge about new technology during the option period without subsequently purchasing licenses.
...
The relationship between technological knowledge and the likelihood of licensing tends to be quite different for companies that purchase options prior to making licensing decisions, however. In such cases, firms that hold patent portfolios concentrated in areas related to the university inventions ( i.e., high-focus firms) are less likely to subsequently license the university patent at the end of the option period. Ziedonis suggests several explanations for this.
Tuesday, January 30, 2007
More on Biotech Generics and the Democratic Congress
Hatch, Republican of Utah , said yesterday that the issue is too important to try to pass before the Senate recess, expected at the end of next week .
``There's no question we can't do much in this Congress," he said. ``I look forward to working with [Waxman], if we can do a balanced approach -- because nothing else is going to pass."
One of the problems is going to be deciding whether or not to put biotech generics through clinical trials of their own. If they're not required to undergo trials, I'm curious to see how they deal with the reality that slightly different drugs will have different effects. Some good, some bad. Who is liable when they're bad, considering there were no trials? This article suggests the burden may be put on the prescribing doctors:
The FDA says it needs similar authorization before it gives copies of biotech drugs speedy reviews. Deputy Commissioner Janet Woodcock says the process would have to be different from the way traditional generic drugs are reviewed. That's because science hasn't advanced far enough for the agency to establish that the biotech copies are identical to the originals - only to say they are similar.
That's why the FDA calls imitations of biotech drugs "follow-ons," not generics. Likewise, the agency wouldn't allow pharmacists to freely substitute follow-on biotech drugs for originals, as they can do with traditional medicines. A doctor would have to specifically request the follow-on, unless the generic drug makers prove through studies that the original and its imitation are the same.
No doubt a lack of pharmacist discretion will mean less generics being used. If you couple that with how direct to consumer marketing campaigns increase prescription rates, it seems biotech companies would be able to defend their market share from generics much better than traditional pharma's can. I would be disappointed but not surprised to see marketing regulations in the bill.
More From India
Hundreds of Indian activists protested in New Delhi on Monday against a challenge to the country's patent law by Swiss pharmaceutical giant Novartis, saying the move could leave millions without access to affordable medicine."Hundreds" doesn't seem like much in India.
Tougher Standards for Biotech
With the number of patents on gene-related discoveries soaring, IP experts say that the process of gaining a patent has become much more complex. Isolating and sequencing a protein won't cut it anymore, say attorneys. You now have to demonstrate a "credible utility" outlining what it can do. Biotech patent applications are considered a prime reason why the total number of patent filings in the U.S. hit 409,500 last year. And many of these patents are out to protect new therapies rather than genetic links to disease.
Pulmonary Arterial Hypertension
Pharmaceutical and biotechnology companies have evinced interest in the PAH markets despite the small patient population. The robust late stage drug pipeline has resulted in in-licensing deals and M&A activity.
Due to a lack of awareness about the disease, the rate of diagnosis and treatment is low, resulting in higher mortality rate among patients diagnosed at a late-stage of the disease.
"The average survival time of patients upon diagnosis is between 3-4 years chiefly due to the low diagnosis rates," says Barath Shankar. "Several patients are unaware of the existence of the disease until progression to a chronic stage.
"Several leading pharmaceutical and biotechnology companies have shown interest in this market. The development of improved therapies, combined with increased awareness is likely to enable the growth of this market
Novartis Patent Case in India
The treatment of thousands of AIDS patients will be in jeopardy if Swiss drug maker Novartis succeeds in changing India's patent law, Medecins Sans Frontieres (MSF) has warned.
Here's a summary time line of Patent Law in India.
Another article puts it this way:
Profit over lifeThey do briefly mention:
In 2000, antiretroviral (ARV) treatment cost was estimated at $10,000 per patient annually. But the availability of generic drugs produced mainly in India, allowed costs to plummet to about $70 per patient per year, Mwangi adds.
Novartis argues that the principle of intellectual property protection must be safeguarded if innovation is to flourish.
Speeding up Tech Transfer
PALM DESERT, Calif., Jan. 30 /PRNewswire-USNewswire/ -- Searching for
the "missing link" to research just got easier. The iBridge(SM) Network, a
program of the Kauffman Innovation Network, Inc., and its accompanying Web
site, debuted today at DEMO 07, the premier launch venue for new products,
technologies and companies. University researchers, industry
representatives, and entrepreneurs can use the iBridge Web site to search
for innovations that, until now, have been lost and untapped behind
university walls.
Here's the iBridge web site: http://www.iBridgeNetwork.org.